Use our HESI chemistry practice test to prepare for your upcoming exam. The practice test is completely free and includes topics you may be tested on.
To practice other subjects, visit our HESI entrance exam practice test home.
Free HESI Chemistry Practice Test
Exam Summary
0 of 15 Questions completed
Questions:
Information
You have already completed the exam before. Hence you can not start it again.
Exam is loading…
You must sign in or sign up to start the exam.
You must first complete the following:
Results
Results
Time has elapsed
You have reached 0 of 0 point(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
Categories
- Not categorized 0%
-
 Keep Studying: You most likely will not pass the HESI chemistry section. You should continue studying. Take some of our full-length practice exams.
Keep Studying: You most likely will not pass the HESI chemistry section. You should continue studying. Take some of our full-length practice exams. -
 Almost There: You are almost there. You may or may not pass the HESI chemistry section, it will be close. Take some of our full-length practice exams.
Almost There: You are almost there. You may or may not pass the HESI chemistry section, it will be close. Take some of our full-length practice exams. -
 You Are Ready: We believe you are ready for the HESI chemistry section. If you would like to continue to practice just to be safe, take some of our full-length practice exams.
You Are Ready: We believe you are ready for the HESI chemistry section. If you would like to continue to practice just to be safe, take some of our full-length practice exams.
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 15
1. Question
CorrectIncorrect -
Question 2 of 15
2. Question
CorrectIncorrect -
Question 3 of 15
3. Question
CorrectIncorrect -
Question 4 of 15
4. Question
CorrectIncorrect -
Question 5 of 15
5. Question
CorrectIncorrect -
Question 6 of 15
6. Question
CorrectIncorrect -
Question 7 of 15
7. Question
CorrectIncorrect -
Question 8 of 15
8. Question
CorrectIncorrect -
Question 9 of 15
9. Question
CorrectIncorrect -
Question 10 of 15
10. Question
CorrectIncorrect -
Question 11 of 15
11. Question
CorrectIncorrect -
Question 12 of 15
12. Question
CorrectIncorrect -
Question 13 of 15
13. Question
CorrectIncorrect -
Question 14 of 15
14. Question
CorrectIncorrect -
Question 15 of 15
15. Question
CorrectIncorrect
Test Your Readiness
You can take our diagnostic exam to see if you are actually ready for your HESI exam. 100% free and includes other subject exams.
HESI Chemistry Overview
The HESI chemistry sections is 1 of 8 total sections on the HESI exam. Odds are you will not be asked to take all 8 sections of the exam.
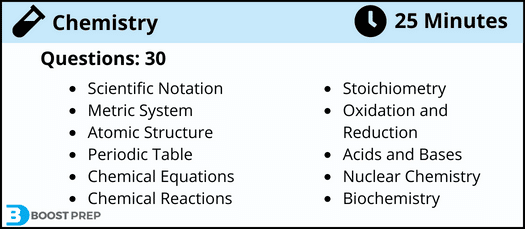
You can expect to find the following on the HESI A2 chemistry section:
- 30 Questions
- 25 Minutes
Some topics you could be tested on include:
- Scientific Notation
- Metric System
- Atomic Structure
- Periodic Table
- Chemical Equations
- Chemical Reactions
- Stoichiometry
- Oxidation and Reduction
- Acids and Bases
- Nuclear Chemistry
- Biochemistry
You can get more free practice by visiting our HESI A2 practice test page.
Studying for HESI Chemistry
When studying for the chemistry portion of the exam, make sure you focus on these concepts:
- Atomic Structure
- Physical Properties of Matter
- Types of Bonds
- Chemical Properties of Matter
- Chemical Equations
- Chemical Reactions
- Chemistry Math
- Solutions and Molarity
- Oxidation
- Acids and Bases
- Nuclear Chemistry
- Biochemistry
Our course covers each of these concepts using learning modules, flashcards, and practice questions.
HESI Chemistry FAQs
There are 30 total questions on the chemistry portion of the HESI. However, there will be 5 unscored questions. You will not know which questions are unscored.
You will have 25 minutes to complete the chemistry portion of the exam. To keep pace, you will want to spend less than 50 seconds per question.
This section is one of the more “technical” sections on the exam. A good way to prepare is by using a HESI test study guide.
A study guide will organize all of the information you need to know in one place. This will save you time and not force you to hunt around for the correct information to study.